Critical properties of linear and branched alkanes
---------------------------------------------- We have developped an equation of state (based on thermodynamic
perturbation theory) to predict the changes in critical properties of
alkanes (linear and branched). In the figure we show the critical
temperature of more than 40 different alkanes, different in
molecular weigth and/or structure (isomers).
The theory is able to account for most of the features found
experimentally.
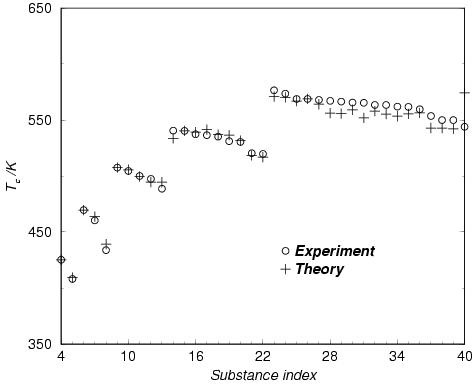
L.G. MacDowell y C. VegaJ.Chem.Phys. 109, 5681 (1998)
Computer simulations of branched alkanes.
----------------------------------------------- We have developped a computer simulation program that allows to
determine the equation of state of any branched alkane. In the figure
the compressibility factor as obtained from computer simulation is
compared to a theoretical equation of state proposed by ourselves
(a modification of Wertheim's TPT1 theory).
Results are shown for several isomers of octane.
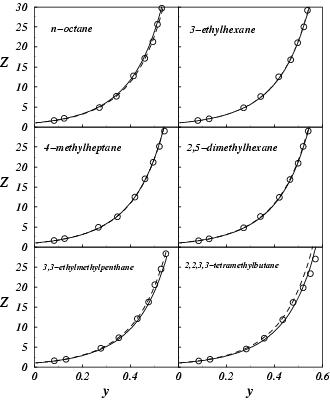
L.G. MacDowell, C. Vega y E. SanzJ.Chem.Phys. 115, 6220 (2001)
Critical properties of polymers
----------------------------------- Equation of state for polymers formed by several tangent
Lennard-Jones monomers. Simulation and theoretical results are
shown. In the figure the chemical potential as a function of
the density is presented for a supercritical isotherm (temperature
larger than the critical temperature) and subcritical isotherm (
temperature lower than the critical temperature).
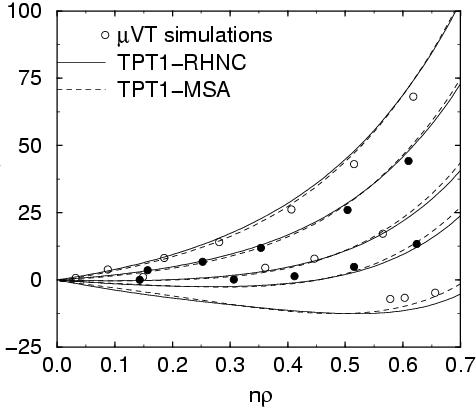
L.G. MacDowell, M. Muller, C. Vega y K. BinderJ.Chem.Phys. 113, 419 (2000)
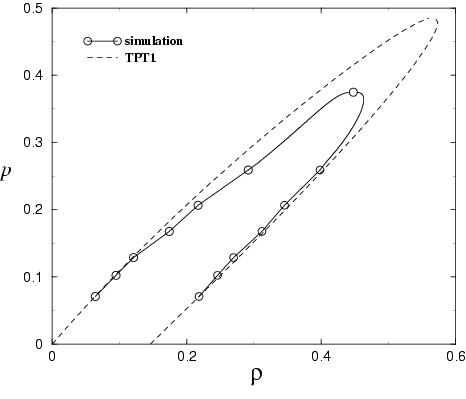
Phase separation of polymer-solvent systems.
------------------------------------------------------- An equation of state for mixture of a long chain (polymer) and
a short chain (solvent) is proposed. In the model the interaction
between monomer units is of LJ type. Simulations for the same model
were performed. In the figure the phase diagram as obtained from theory
and from simulation is presented (in the pressure-composition plane
and in the pressure-density plane).
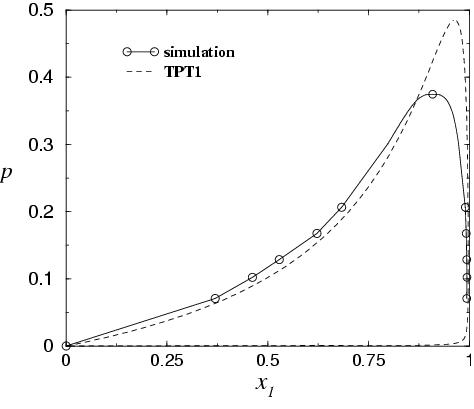
L.G. MacDowell, P. Virnau, M. Muller, K. BinderJ.Chem.Phys. 117, 6360 (2002)